Our team is developing thermochemical material (TCM)-based thermal energy storage. In a TCM, energy is stored in reversibly forming and breaking chemical bonds. TCMs have the fundamental advantage of significantly higher theoretical energy densities (200 to 600 kWh/m3) than phase change materials (PCMs; 50 to 150 kWh/m3). They also exhibit negligible self-discharge because the energy is stably locked up in chemical bonds, making them uniquely suited as compact, stand-alone solutions for daily-seasonal energy storage in buildings. TCMs can be used as a thermal battery, charging with solar energy or excess grid electricity and discharging to supply thermal end-uses in buildings such as space and water heating.
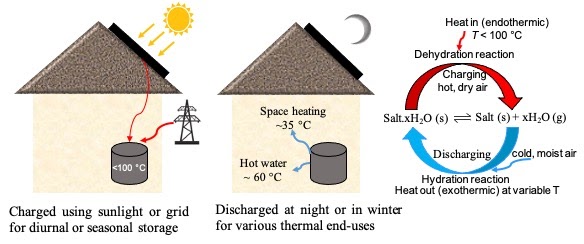
Although promising, the available research shows TCM-based storage suffers from instabilities both at the material and reactor level resulting in poor multi-cycling efficiency and a high levelized cost of thermal energy storage. Our aim is to fundamentally investigate TCMs to overcome these challenges by developing new design rules at both the material and reactor level. This project seeks to bridge the gap between the high theoretical storage potential of thermochemical salt hydrates (>600 kWh/m3) and their sub-par performance when integrated into real thermochemical reactors for energy storage with repeated cycling (<70 kWh/m3, and fewer than 20 cycles). This project is funded by the DOE’s Building Technology Office, and is in collaboration with Prof. Chris Dames in UC Berkeley, NREL and NET Energy Inc.